How Did They Use Atoms to Explain Different Physical Properties
Add your answer and earn points. How did they use atoms to explain physical properties.

1 4 Classification And Properties Of Matter Chemistry Libretexts
When a substance has different.
. Go to httpgalileophysvirginiaeduclasses252atomshtml and read the section on Early Greek Ideas in order to answer the following questions. How did they use atoms to explain different physical properties. Atoms are indivisible and indestructible.
3 Compounds are formed by a combination of two or more different kinds of atoms. Dalton publish is Atomic Theory in 1808. What do atoms use to bond with other atoms.
When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different. In terms of chemical and physical properties. 1 All matter is made of atoms.
By looking at the chemical properties and physical properties of the elements next to a gap he could also predict the properties of these undiscovered elements. When did Dalton develop his atomic theory. Atomic Theories and Models Answer these questions on your own USING COMPLETE SENTENCES where.
Chemical properties of different isotopes are almost similar. 2 All atoms of a given element are identical in mass and properties. Want this question.
2Atoms are indivisible and indestructible. How were the ideas of these two men received by. To explain the gaps Mendeleev said that the gaps were due to undiscovered elements.
The atoms are arranged in layers. Early Ideas About Atoms. However Mendeleev was never able to explain why some of the elements were out of order or why the elements should show this periodic behavior.
The element germanium was discovered later. What was the basic idea about matter that Leucippus and Democritus proposed. Chemistry is the discipline that studies these changes.
Scientists use a particle accelerator to smash light atoms into a thin metallic foil that contains heavier atoms. 4 A chemical reaction is a rearrangement of atoms. 1 All matter is made of atoms.
Mendeleev left gaps in his table to place elements not known at the time. Note that today we know atoms of the same element can have different masses. And for atoms to bond they must do at least one of the following.
In fact his table successfully predicted the existence of gallium and germanium which were discovered later. Learn about the structure of the atom and how atoms make up matter. Atoms can be combined into find ratios reform different compounds.
The different physical properties color and taste etc of materials came about because atoms in them had different shapes and arrangements and orientations with respect to each other. Lose electrons to other atoms. Atoms of the same element with different masses are usually called isotopes.
View Webquest_ Atomic Theories and Modelspdf from CHEM MISC at Spring Creek High. Here is a model showing atoms of different elements with. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature.
They hope that the two nuclei at the centre of these atoms will fuse and form a. 3 Compounds are formed by a combination of two or more different kinds of atoms. 4 A chemical reaction is a rearrangementof atoms.
Isotopes of an element have different physical properties because they have different mass numbers. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. And recall that valence electrons occupy the highest energy orbital in an atom.
The Periodic Table can predict the properties of new elements because it organizes the elements according to their atomic numbers. Share electrons with other atoms. Explain the physical properties of isotopes - 32055402 DreamGirl54 DreamGirl54 28122020 Chemistry Secondary School answered Explain the physical properties of isotopes 1 See answer Advertisement Advertisement DreamGirl54 is waiting for your help.
Atoms they are arrange diffrently A combination of two or more atoms that has physical and chemical properties that differ from the atoms that compose it is called. The physical properties of any isotope are largely determined by its mass. While atoms of different elements have different mass and the mass of these atoms remain the same after a physical or chemical change.
The greater the force needed the harder and stronger the metal. Contents 1 History of atomic theory 11 In philosophy. Lastly chemical reactions are able to cause atoms to separate from one another combined with each other or rearrange in terms of their bonding.
Add an answer. An atom is the smallest unit of matter that retains all of the chemical properties of an element. When a force is applied the layers may slide over each other.
How did they use atoms to explain different physical properties. This would have to wait until we knew. How did Mendeleev predict undiscovered elements.
Atoms use their valence electrons to bond with other atoms. Atoms from one element are observed to be different than other elements. Gain electrons from other atoms.
Atoms and Atomic Structure. Creating new elements is not a simple process.
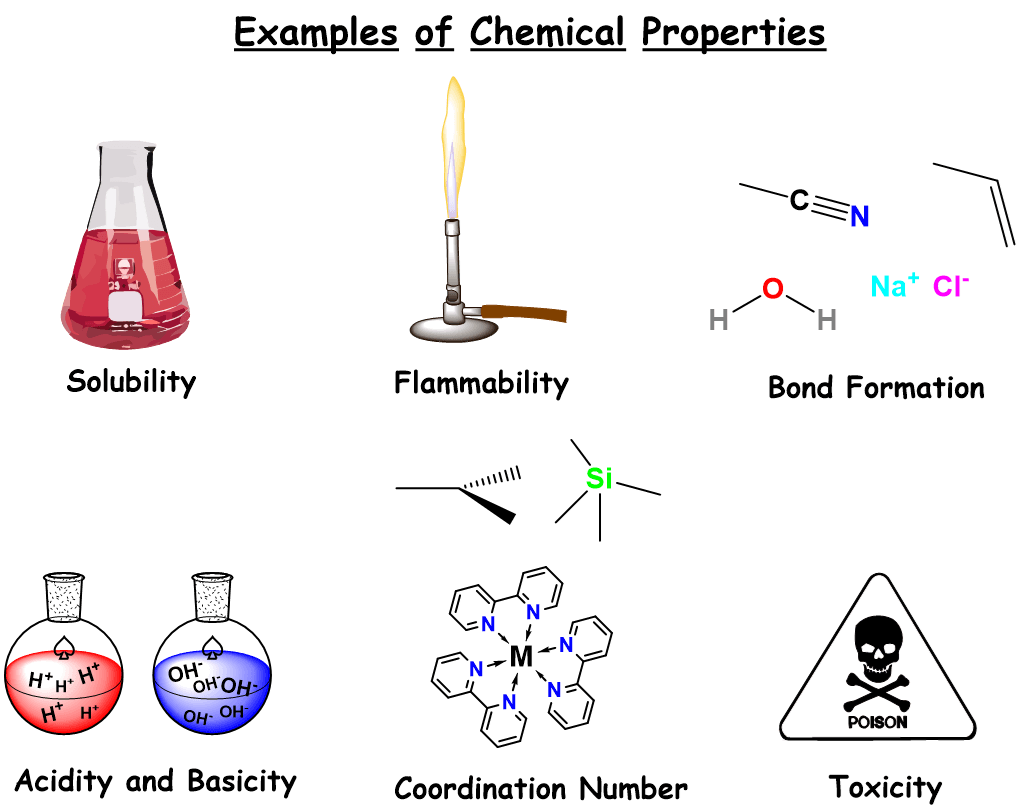
Physical Vs Chemical Properties Definition Examples Expii

Learn About Physical Properties Chegg Com

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table

1webquest Scientists Atom Answers Converted 1

Webquest Atomic Theories And Models

Physical Properties Of Elements
Describing And Classifying Matter Let S Talk Science
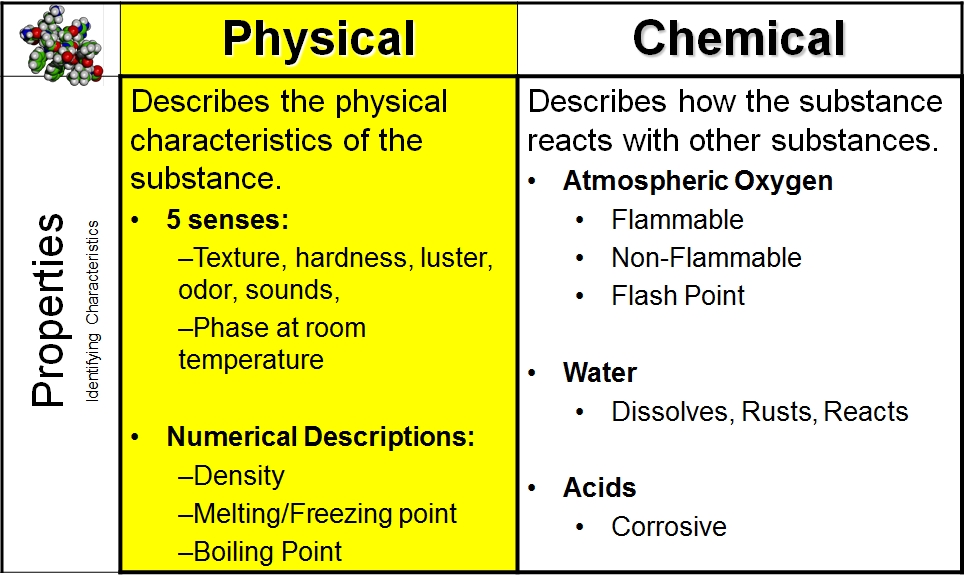
Chemical Physical Properties Vista Heights 8th Grade Science
History Of Atomic Theory Webquest

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table

Learn About Physical Properties Chegg Com
Describing And Classifying Matter Let S Talk Science

Physical Vs Chemical Properties Youtube

Physical Chemical Properties Of Matter Density Melting Point Boiling Point Hardness Electric Thermal Conduction Science Online

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table

3 Ways To Study The Chemical And Physical Properties Of Atoms In The Periodic Table

Webquest Atomic Theories And Models

Physical Property Of Matter Definition Examples Video Lesson Transcript Study Com
Comments
Post a Comment